Introduction: The Accountability Challenge in MLR Review
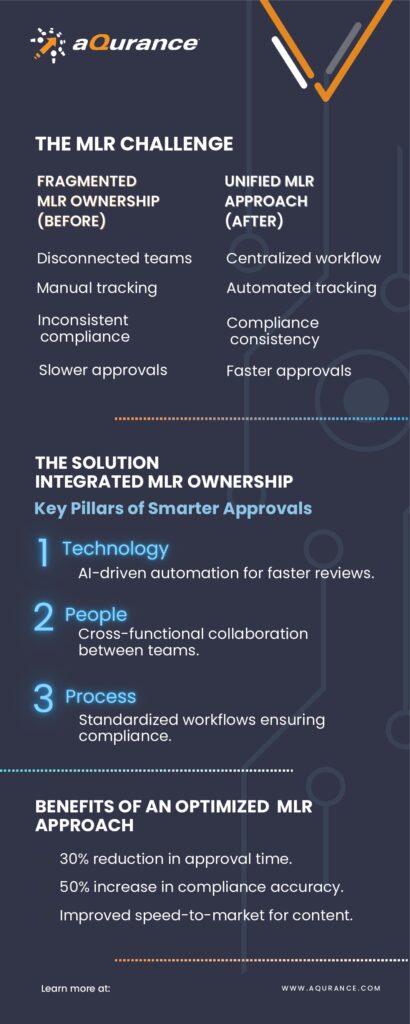
Medical, Legal, and Regulatory (MLR) review processes are pivotal in ensuring compliance, efficiency, and speed-to-market. Yet, despite advances in technology and process optimization, many organizations still encounter delays, inefficiencies, and inconsistencies—not because the right tools don’t exist, but because accountability is distributed without clear ownership.
According to EY’s research on MLR optimization, companies spend up to 40% more time than necessary on review cycles due to unclear roles, redundant reviews, and process misalignment. With regulatory scrutiny intensifying and the need for agility in content approvals growing, leaders must rethink how they align technology, teams, and workflows to eliminate bottlenecks.
The Three Dimensions of MLR Efficiency: Technology, People, and Process
To resolve inefficiencies and drive operational excellence in MLR, organizations must address three interdependent dimensions:
- Technology: Enhancing MLR Efficiency Through Smart Governance
Advanced platforms such as Veeva Vault PromoMats and AI-powered compliance tools are transforming the industry, yet technology alone cannot solve structural inefficiencies. In many cases, the lack of strategic governance within these systems results in:
- Inconsistent application of review criteria, leading to unnecessary escalations
- Technology underutilization, as automation and AI tools are bypassed due to uncertainty in decision-making
- Limited process visibility, making it difficult to track and optimize review cycle performance
🔹 Strategic Insight: Companies that integrate structured governance frameworks within their MLR platforms experience greater process control and improved cycle times. Aqurance has helped organizations streamline workflows within Veeva, ensuring automation and AI are aligned with clear decision-making processes to reduce review bottlenecks.
- People: Aligning Cross-Functional Teams for Seamless Execution
While MLR is inherently a cross-functional process, misaligned priorities across marketing, regulatory, and legal teams can slow progress. Common friction points include:
- Marketing teams striving for speed and agility while
- Regulatory teams focus on compliance, leading to over-reviewing to mitigate perceived risks
- Legal’s risk-averse stance, which can introduce additional layers of scrutiny
Without a structured approach to accountability, these competing priorities often create review inefficiencies that impact commercial timelines.
🔹 Strategic Insight: Rather than introducing another layer of oversight, organizations benefit from centralized MLR coordination models. Aqurance has worked with life sciences leaders to establish structured MLR governance, improving collaboration between teams while ensuring compliance without unnecessary delays.
- Process: Balancing Standardization with Flexibility
MLR review processes must strike a balance between efficiency and regulatory rigor. However, rigid, one-size-fits-all approaches can slow down approvals for lower-risk content, while fragmented processes result in compliance inconsistencies across markets.
🔹 Strategic Insight: A risk-based MLR review framework enables organizations to prioritize high-risk content for deeper evaluation, while streamlining approvals for lower-risk materials. According to industry research, companies implementing tiered review models see a 30% improvement in approval times without compromising compliance. Aqurance has helped clients implement structured risk-based models, enabling smarter resource allocation and accelerating market readiness.
The Leadership Imperative: How to Drive MLR Excellence
Achieving true MLR efficiency requires an approach that aligns technology, teams, and processes under a unified strategy. Key leadership actions include:
![]() Embed governance within MLR technology—Ensure automation, AI, and review workflows are structured with clear decision pathways and real-time visibility.
Embed governance within MLR technology—Ensure automation, AI, and review workflows are structured with clear decision pathways and real-time visibility.
![]() Enable cross-functional alignment—Create structured collaboration frameworks to ensure marketing, regulatory, and legal teams operate with a shared understanding of priorities.
Enable cross-functional alignment—Create structured collaboration frameworks to ensure marketing, regulatory, and legal teams operate with a shared understanding of priorities.
![]() Implement risk-based review models—Optimize resource allocation by ensuring appropriate oversight levels based on content risk.
Implement risk-based review models—Optimize resource allocation by ensuring appropriate oversight levels based on content risk.
![]() Leverage data for continuous improvement—Use analytics to track cycle times, identify process inefficiencies, and refine workflows over time.
Leverage data for continuous improvement—Use analytics to track cycle times, identify process inefficiencies, and refine workflows over time.
Conclusion: Turning MLR Efficiency into a Competitive Advantage
In today’s fast-evolving regulatory landscape, MLR is no longer just a compliance function—it is a strategic enabler of commercial success. By addressing inefficiencies in ownership, technology governance, and process alignment, life sciences organizations can transform MLR from a bottleneck into a streamlined, scalable advantage.
With deep expertise in MLR workflow optimization and digital transformation, Aqurance partners with life sciences leaders to implement practical, high-impact solutions that drive measurable improvements in review efficiency, compliance integrity, and speed-to-market.
Industry References:
EY Smart Reviewer – AI-Driven MLR Optimization
Veeva: Future of MLR Review Efficiency – Best Practices for Automation & Collaboration
Gartner: Digital Transformation in Pharma – Strategic Technology Adoption
Want to Learn More? contact us today.




